MSc Student
Advisor: Georg Brenneis
Unit for Integrative Zoology, Department of Evolutionary Biology
University of Vienna
Abstract
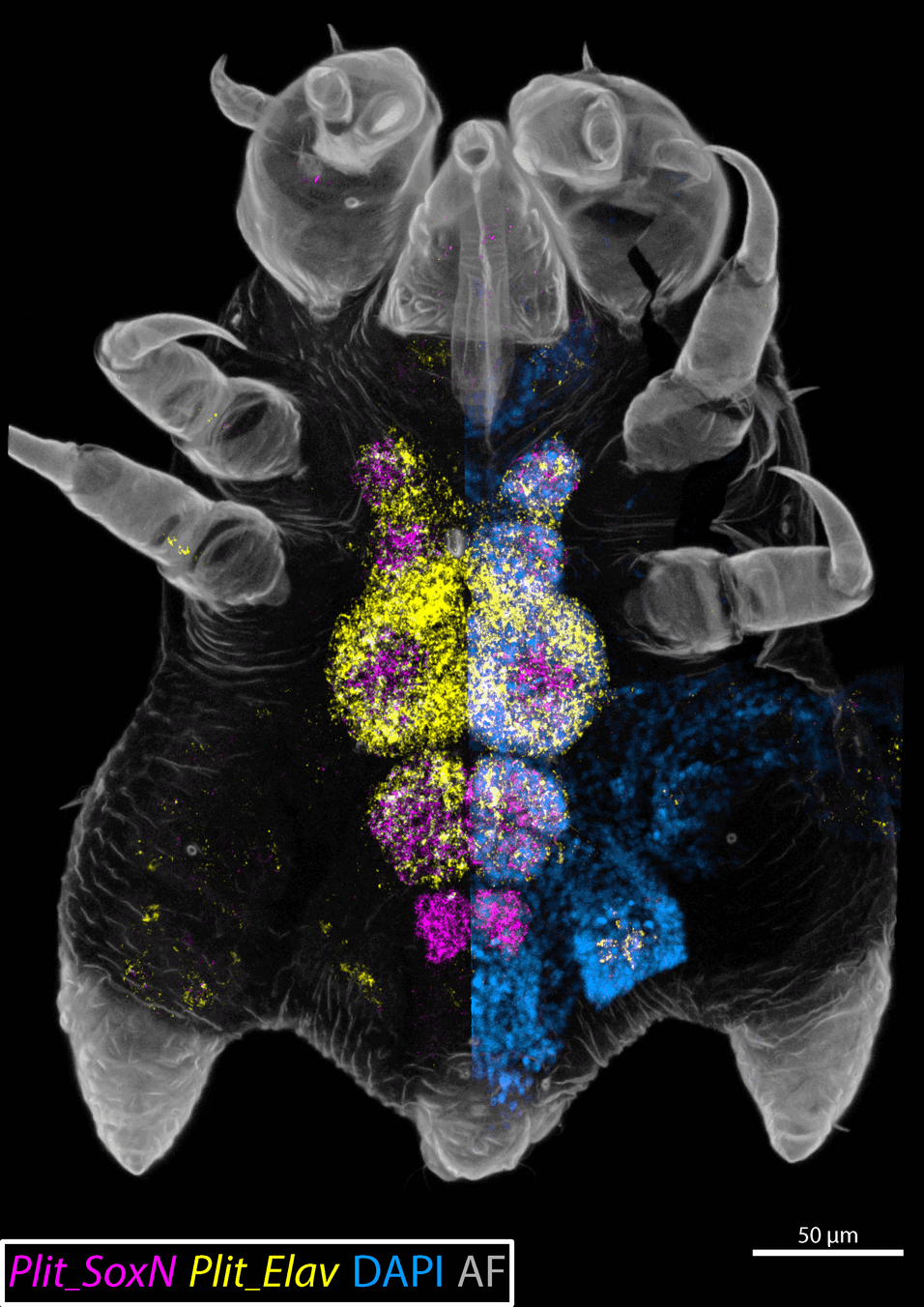
Pycnogonida (sea spiders) is a group of bizarre marine arthropods, which are widely accepted as a member of the Chelicerata (true spiders, scorpions and kin), in which they represent the sister group of all remaining extant taxa (termed Euchelicerata). Interestingly, the development of the nervous system, i.e. neurogenesis, in pycnogonids differs from that of other chelicerates in that it appears to feature large neural stem cells (NSCs) which show high mitotic activity as well as a morphologically asymmetric mode of division. These cells show many similarities with neuroblasts (NBs), a well-characterized type of neural stem cell driving neurogenesis in the Pancrustacea (crustaceans + Hexapoda). Neuroblasts also divide asymmetrically resulting in one daughter cell retaining NB identity while the other assumes the identity of a so-called ganglion mother cell (GMC), which in turn divides typically only once and symmetrically, producing two post-mitotic neurons/glial cells. In pycnogonids, similar cells, which are morphologically smaller than the NSCs and divide symmetrically, have previously been described and termed intermediate neural precursor (INP).
Unlike that of pancrustaceans, the underlying gene regulatory network of pycnogonid neurogenic cell types has not been investigated prior to this study. Accordingly, my thesis provides first insights into the transcriptional profiles of cells involved in neurogenesis of Pycnogonum litorale, furthering the understanding of these understudied cell types.
While the expression patterns of several genes with crucial roles during arthropod neurogenesis were found to be mostly consistent between pycnogonid NSCs/INPs and pancrustacean NBs/GMCs, too many dissimilarities and incongruencies remain to satisfactorily conclude the postulated homology of pycnogonid and pancrustacean neurogenic cell types.
Further it was shown that the first cells adopting neural fate (neural precursors) are specified in segmentally stereotypic positions in the neuroectoderm. Additionally, the data points to the lack of a previously described initial phase, featuring immigrating post-mitotic cells before the differentiation of NSCs, during postlarval neurogenesis of P. litorale.